
Suzanne M. Dugan
As pension funds across the country put 2023 behind them, the new year may bring additional headwinds. (Keep in mind: 2024 is a leap year, so there is one more whole day this year for complication and challenge!) Concerns about interest rates and inflation are front of mind for institutional investors, who are wondering whether the Federal Reserve will cut interest rates and how much the economy will slow. A presidential election year brings further uncertainty. Beyond those concerns, here are some key areas that public pension plan leaders have said they will be thinking about in the 366 days of 2024.
Cybersecurity
Managing cybersecurity risk will be a top priority in 2024. The U.S. recorded a 75% increase in ransomware events between July 2022 and June 2023, according to Malwarebytes, Inc. The National Conference on Public Employee Retirement Systems notes that public employee pension funds are prime targets for cyber criminals drawn by the fact that they collect large amounts of personally identifiable information, hold significant assets, and have relatively small staffs. Any doubt about this was resolved in June 2023, when the nation’s two largest US pension plans, CalPERS and CalSTRS, were involved in a worldwide data security incident that impacted one of their contracted third-party vendors. The so-called MOVEit hack, named after the popular file transfer software that was breached, demonstrates that pension plans must be cognizant of their fiduciary risk. As the U.S. Department of Labor has emphasized, plan fiduciaries have an obligation to ensure proper mitigation of cybersecurity risks. One mitigation risk tool that pension systems have begun instituting are cybersecurity tabletop exercises, which simulate real-world attacks and are designed to test the organization’s ability to respond to a cybersecurity incident.
Artificial Intelligence
The use of artificial intelligence (AI) is another hot topic in the pension plan world in 2024. The CFA Institute noted in a report issued in October 2023 that “the potential impact of AI on the pensions industry is likely to be widespread.” In a webinar hosted by the National Institute on Retirement Security, Andrew Roth, the Deputy Director of the Teacher Retirement System of Texas, observed that “tools that have AI components built into them [have] great promise for transformational technology to quickly get things done and do things faster with fewer resources” but “underlying that promise is a lot of risk.” Pension systems are exploring the use of AI in a wide variety of ways, such as plan operations, member communications, retirement planning, investment analysis, and modeling. The CFA Institute notes that as pension systems learn how to integrate AI into their processes, each decision must be considered through an ethical lens. The report finds that AI can be used in many aspects of pension systems to potentially improve returns and reduce costs, “thereby delivering a higher standard of living in retirement—a worthwhile objective for all pension systems.” But as the CFA Institute notes, “[a]ctive governance and clear accountability are essential in the development of all AI models and algorithms” and “[t]his will require experienced pension professionals to be involved, for, without that experience, judgment and oversight, there is the real risk that some outcomes will be helpful or misleading, or possibly even wrong, in the complex world of pensions.”
Governance
There are a myriad of other key challenges that pension plans face in 2024—from regulatory issues (IRS guidance on Secure 2.0) to litigation (seeking to overturn the new SEC rule requiring increased disclosure from private fund advising and prohibiting certain fee arrangements) to politicization (efforts to prohibit pension plans from making certain investments, or from doing business with certain investment managers). As the Council of Institutional Investors wrote in a recent letter, it believes “the heightened political atmosphere of U.S. elections will increase public scrutiny of members’ investment policies and practices—especially those related to sustainability.” An overarching principle that stands out when pension plans are addressing issues like these with fiduciary implications is the need for good plan governance. As noted by the Stanford Institutional Investors’ Forum Committee on Fund Governance, just as good organization governance is critical to publicly owned corporations (corporate governance), it is also critical to pension plans that own the stocks of those companies (plan governance). It cannot be said strongly enough: governance matters. It reduces the risk of conflicts of interest, abuse of authority, and misuse of plan resources. It helps ensure organizational performance, such as proper payment of benefits, and multiple studies have concluded that governance is, in fact, a key driver of strong investment performance—which is necessary to pay benefits. Good governance can also help attract and retain employees to public pension plans, which may not be able to compete with private sector salaries but can win employees’ hearts and minds through their mission to protect the retirement security of the nation’s teachers, safety officers, and other public servants. In 2024, more than ever, sound governance results in greater transparency, promotes buy-in from plan sponsors, legislators and other stakeholders, and enables trustees and administrators to fulfill their fiduciary duty to the members and beneficiaries of their pension plans.
Richard E. Lorant
A federal judge in Utah has certified a class of Pluralsight, Inc. investors seeking damages after Pluralsight stock dropped 40% when executives allegedly admitted they had exaggerated the size of the sales force key to the company’s continued growth.
In a December 27 memorandum decision and order granting class certification, U.S. District Judge David Barlow designated lead counsel Cohen Milstein as class counsel and its clients— lead plaintiffs Indiana Public Retirement System and Public School Teachers’ Pension and Retirement Fund of Chicago—as class representatives.
“We are very pleased with this detailed and well-reasoned opinion,” said Carol V. Gilden, the Chicago-based partner leading Cohen Milstein’s litigation team. “With the class certified, we can focus on marshaling the evidence we are collecting through the discovery process to secure the best possible resolution for our clients and the class.”
Headquartered in Utah, Pluralsight provides cloud-based and video training courses, skill and role assessments, learning paths, and analytics tools to businesses. Plaintiffs allege that the company and two top executives violated securities laws by making materially false misrepresentations and omissions about Pluralsight’s sales force and its ability to sustain strong growth in billings.
The complaint further accuses the executives, Aaron Skonnard, the CEO and Chairman, and James Budge, the chief financial officer, of violating securities laws by trading stock based on their inside knowledge. In all, plaintiffs allege that Pluralsight’s top three executives sold $47 million in stock during the class period, which runs from January 16, 2019, through July 31, 2019, including through their 10b5-1 trading plans.
In his opinion, Judge Barlow found that plaintiffs satisfied the requirements to pursue a class action under Federal Rule of Civil Procedure 23(a). Under the rule, the class must be large enough to make it impractical to pursue claims as individuals; the class members must share common “questions of law or fact;” and the class representatives must have “claims or defenses” typical of those of the class at large and “fairly and adequately” protect the interests of the class.
In appointing class counsel, the judge found that “Cohen Milstein will fairly and adequately represent the class’s interests.” He based his decision on the firm’s prosecution of the lawsuit since its March 2020 lead counsel appointment, its experience as class counsel in other cases, and its significant resources.
Indeed, it took plenty of perseverance and skill to even reach the class certification stage. Filed in New York, the proceedings were transferred to the District of Utah where, in March 2021, the judge who was first assigned to the case dismissed plaintiffs’ amended complaint. More than a year later, in August 2022, the Tenth Circuit Court of Appeals reversed the lower court’s dismissal on plaintiffs’ main claims. Following their successful argument to the appeals court, lead plaintiffs filed their second amended complaint in November 2022 and followed with their motion to certify the class in March 2023.
The case is Indiana Public Retirement System, et al. v. Pluralsight, Inc. et al., 19-cv-00128- DBB-DAO (D. Utah).
Carol V. Gilden and Laura H. Posner
Fraud by omission versus commission. Should a corporation be able to do one but not the other in its mandatory discussion of known trends without risking liability under Section 10(b) of the Securities Exchange Act? This is a question the Supreme Court has been itching to answer.
The case is Macquarie Infrastructure Corp. et al. v. Moab Partners LP et al., case number 22- 1165. Back in 2017, the Supreme Court was prepared to review the issue in another case, Leidos Inc. v. Indiana Public Retirement System et al., case number 16-581, but the case settled a month before arguments were scheduled. This time, there don’t appear to be any settlements on the horizon, and numerous parties, including the U.S. Solicitor General, have filed amicus briefs, signifying the high stakes involved.
Important to investors is an SEC disclosure requirement under Regulation S-K Item 303, 17 CFR section 229.303 (“Item303” disclosures, also known as Management’s Discussion and Analysis of Financial Condition and Results of Operations), which requires companies to disclose “where a trend, demand, commitment, event or uncertainty is both presently known to management and reasonably likely to have material effects on the registrant’s financial conditions or results of operations.” The purpose, according to the SEC, is to enable investors “to assess the financial condition and results of operations” of a company and its “prospects for the future.”
In the case under review, Macquarie did not disclose that one of its most profitable subsidiaries was about to be subject to a United Nations regulation limiting pollution that would significantly eat into its profits. The plaintiff’s 2018 lawsuit claims the defendants concealed the pending restrictions for two years. When the company finally did disclose the limitations it faced, its stock fell by over 40%.
The defendants argue that even if they had a duty to disclose the expected impact of the United Nations regulations under Item 303, they should not be held liable for failing to do so under Section 10(b) of the Securities Exchange Act. The district court sided with the defendants, but a unanimous Second Circuit disagreed and reinstated the claims in December 2022 before the Supreme Court ultimately agreed to review the case in September 2023.
Considering the high stakes involved for investors, who could see their ability to recover losses through private actions severely limited, Cohen Milstein has been actively engaged in the amicus effort to support the plaintiffs in the case and to respond to the arguments raised in amicus briefs filed in support of defendants by heavyweights like the U.S. Chamber of Commerce and Securities Industry and Financial Markets Association (“SIFMA”).
This amicus effort includes briefs filed on behalf of dozens of securities law and business professors, institutional investors with over 340 billion in assets under management, and a group of consumer advocates who include the Consumer Federation of America, Better Markets, Inc., Public Justice, and the American Association for Justice.
As part of that amicus effort, Cohen Milstein authored an amicus brief on behalf of former SEC Commissioners and senior officials appointed by both Republican and Democratic presidents. That brief addressesthe defendants claim that allowing for Section 10(b) liability for violations of Item 303 will force companies to provide overbroad and unnecessary disclosures that will confuse investors. Cohen Milstein’s clients noted in their amicus brief that the SEC has “repeatedly highlighted that Only material items” be included in such disclosures, and that the SEC “expressly condemned unnecessary or duplicative disclosures precisely because they frustrate investor understanding.” Indeed, in its 2003 Guidance, the SEC encouraged companies to “de-emphasize (or, if appropriate, delete) immaterial information that does not promote understanding.”
The former SEC officials brief also noted the crucial role private actors play in the enforcement of securities laws, which ultimately provide investor confidence that promotes the liquidity of the U.S. securities market to the benefit of corporations and investors alike. The U.S. securities markets would not be “the envy of the world” without strong enforcement mechanisms, of which private actors are a vital part.
The Supreme Court has recognized this role as well, finding that private securities fraud actions provide “a most effective weapon in the enforcement” of securities laws and are “a necessary supplement to [SEC] action.” J.I. Case Co. v. Borak, 377 U.S. 426, 432 (1964). The former SEC officials’ brief noted that the “commission and its senior leadership have repeatedlyinformed this Court of its view that private actions serve an essential role.” As then-Chairman Richard Breeden explained in testimony before the US Senate, the SEC “does not have adequate resources to detect and prosecute all violations of the federal securities laws,” private actions thus “perform a critical role in preserving the integrity of our securities markets.”
The brief also discussed how the SEC has long recognized that a violation of Item 303 can serve as a basis for a Rule 10b-5 action and rejects the defendants’ argument that fraud by omission should be permitted while fraud by commission should not. It is no surprise, therefore, that the plaintiffs were joined by the Solicitor General, who not only filed a brief in support of the plaintiffs but also asked to be allowed to make oral arguments. The Supreme Court granted this request on January 5, 2024, and oral arguments in the case took place January 16, 2024. Cohen Milstein will continue to closely monitor the case to ensure investor interests are protected.
1 Commission Statement About Management’s Discussion and Analysis of Financial Condition and Results of Operations, 67 Fed.Reg. 3746 at 3747 (Jan 25, 2002).
When Congress passed the Securities and Exchange Act of 1934, one of its main goals was to protect the marketplace from the kind of manipulative conduct that precipitated the Great Wall Street Crash of 1929. In the nine decades since, technology has evolved tremendously, and with it the methods devious traders use to manipulate stock prices. But the fundamental threat market manipulation poses to the integrity of securities markets remains unchanged. That’s why Cohen Milstein has developed a series of innovative cases to hold trading firms and individuals accountable when they engage in manipulative securities transactions.
In a class action on behalf of investors in XIV notes, for example, the firm alleged that Credit Suisse manufactured a crash in these securities to obtain illegal profit and we obtained a groundbreaking decision from the Second Circuit holding that these allegations sufficiently pled market manipulation claims. We also represent a class of shareholders in Overstock who allege that the company’s “short squeeze” manipulated the market for its own securities; those claims are currently under review by the Tenth Circuit. And when the Supreme Court considered the scope of key market manipulation provisions of the Exchange Act, we filed an amicus brief advocating for the position that the Court ultimately adopted in holding a broker liable for engaging in manipulative conduct.
Most recently, we filed two market manipulation lawsuits on behalf of dynamic companies in the biotech and information technology industries against some of the nation’s largest broker-dealers for allegedly manipulating the price of these companies’ shares for their own profit. The cases allege that the defendants engaged in “spoofing” to artificially drive down the price of the companies’ shares in order to purchase them at below-market prices.
Spoofing is a form of market manipulation that typically involves placing large “baiting” orders on one side of the market to induce other traders to follow suit, then buying or selling that security on the other side of the market at the artificial prices created by the spoofing, and finally cancelling the baiting orders before they are executed.
The particular mechanisms of spoofing can involve complex features of high-frequency trading algorithms in electronic trading venues. But the basic concept can be analogized to a headfake in sports. A trader fools the marketplace into thinking it is trading in one direction with the goal of moving other traders in that direction, allowing the trader to execute its true trading intention in the other direction, at a greater profit. In our two cases, we allege that the defendants wished to purchase the companies’ shares at artificially low prices and used baiting orders to sell in order to execute buy orders at better prices.
Spoofing in the age of high-speed trading has been prosecuted criminally and civilly by the Department of Justice, Securities and Exchange Commission, and Commodities Future Trading Commission. But private spoofing cases have been very rare. This is in part because government agencies, unlike private plaintiffs, have access to pre-suit investigative discovery tools to obtain and analyze nonpublic trading data.
In our cases, we responded to this challenge by conducting comprehensive and sophisticated analysis of multiple sources of publicly available trading data, matching orders and executions, and applying parameters to identify patterns that courts have held to be indicative of spoofing. These patterns include placing large baiting orders on the opposite side of the market from smaller legitimate orders, cancelling the baiting orders after the smaller orders have executed, leaving the baiting orders on the market for only a short period of time, placing baiting orders behind other legitimate orders to make them less likely to execute, and other conduct contrary to acting as an ordinary market maker.
In both of our spoofing cases, defendants have moved to dismiss the complaint. In the Northwest Biotherapeutics case, briefing has concluded, and oral argument was held on November 14, 2023 before Magistrate Judge Gary Stein in the Southern District of New York. Arguing for the plaintiffs, we explained how our allegations are exactly the type that courts have consistently held sufficient to plead spoofing claims. The defendants argued, as those accused of spoofing always do, that their conduct was normal trading activity, either making markets or trading on behalf of clients. Magistrate Judge Stein recently issued a report and recommendation that agreed with our position on the sufficiency of our allegations as to defendants’ manipulative conduct, scienter, and reliance, and concluded that only our loss causation allegations require more detail in an amended complaint. We await final orders from the district court judges in both cases.
Favorable decisions affirming the sufficiency of these complaints would be a major development towards fairer markets and remedies for companies and investors that have been victimized by manipulative trading schemes.
Every five years, the U.S. Equal Employment Opportunity Commission sets forth a strategic enforcement plan, or SEP, setting priorities that inform how it deploys its limited resources.
The most recent iteration of this plan was published on Sept. 21, 2023, for the purpose of focusing and coordinating the commission’s work over the course of multiple years. Its final version is the product of rigorous public engagement, including several dedicated public listening sessions featuring stakeholders with varied experience and perspectives on the issues that affect the EEOC’s mandate to enforce federal anti-discrimination laws.
The SEP is distinct from the commission’s strategic plan. Where the latter outlines operations and processes the agency will implement to reach its goals, the SEP names its highest-priority subjects for enforcement.
The commission’s framing of these priorities — the way it places its mandate to combat workplace discrimination in the context of broader social movements and the fight for justice and equality — can offer insight into how commission leadership is thinking about resource allocation and enforcement priorities over the covered period.
Among the priorities highlighted in this SEP are a set dedicated to preserving access to the legal system. The issues specifically enumerated in this section would “limit substantive rights, discourage or prohibit individuals from exercising their rights under employment discrimination statutes,” or impede the commission’s enforcement work.
Preserving access to the legal system was part of the EEOC’s first SEP, covering fiscal years 2013-2016, and it appeared again as a subject matter priority in the 2017-2021 version. While its inclusion in the latest SEP is not novel, the composition of the EEOC changed prior to this iteration of the plan.
With the confirmation of Democratic-appointee Commissioner Kalpana Kotagal in August 2023, and confirmation of Chair Charlotte Burrows for a new term in November 2023, the EEOC will have a Democratic majority through at least the remainder of the Biden administration. Additionally, a new general counsel, Karla Gilbride was confirmed in October 2023 to helm the commission’s litigation strategy.
With experience as litigators and advocates, Kotagal and Gilbride know how anti-discrimination laws work in practice; both add their own understanding and knowledge of the subject matter priorities to the existing commission members’ expertise.
We can glean some insights on several of the issues highlighted in the plan from prior public commentary by the commission’s leadership — in particular from these two new leaders. A review of this background is below and should contribute to an overall understanding of where and how the agency will approach select policies and practices identified in the SEP as they relate to access to justice.
This article examines the EEOC SEP’s three highest priorities dedicated to preserving access to the legal system:
- Unlawful, Unenforceable or Improper Arbitration Agreements
- Employers’ Failure to Keep Data and Records Required by Statute or EEOC Regulations
- Retaliatory Practices That Detrimentally Affect Employees
Following the Federal Trade Commission’s 2021 publication of “Nixing the Fix: An FTC Report to Congress on Repair Restrictions,” private “right to repair” cases have multiplied against companies that leverage their market power in a “primary equipment market” (e.g., tractors) to force their customers also to purchase their offerings in “aftermarkets” (e.g., tractor repairs) that otherwise would be competitive. In this article, Daniel McCuaig argues that the application of the 1992 Supreme Court decision in Eastman Kodak Co. v. Image Technical Services, Inc. to these cases misunderstands that case and improperly shields monopolists from competitive pressures, including in Epic’s recent case against Apple.
Can you commit securities fraud by tweeting an emoji? One court confirmed that you can in an important recent decision from the District Court for the District of Columbia.
In Bed Bath & Beyond Corporation Securities Litigation,1 Judge Trevor McFadden held that the plaintiffs had adequately alleged multiple securities fraud, insider trading, and market manipulation claims against Ryan Cohen.
Defendant Cohen is an entrepreneur-turned-investor who founded the online pet store Chewy and sold it for more than $3 billion. Most recently, Cohen became an investor in so-called “meme stocks.” These stocks are popular among retail investors who gather online on Twitter (now known as “X”) and Reddit, often using memes and emojis to discuss their trades (thus the moniker “meme stock”). Meme stock traders are known for buying and selling stocks of companies that most traditional investors either ignore or short (that is, bet that the price will fall rather than rise).
Cohen entered the meme stock fray in 2020 by buying a large stake in GameStop, the struggling brick-and-mortar video game retailer. After buying his stake in GameStop, Cohen made multiple business recommendations and soon selected several directors of its board. GameStop had been popular with meme stock investors, but when they found out about Cohen’s involvement, GameStop’s stock soared by more than 40%. Cohen’s popularity rose and he was soon viewed as the leader of meme stock investors, with media outlets naming him the “meme stock king.”
Cohen followed the same playbook with the struggling retailer Bed Bath & Beyond. In early 2022, Cohen bought a 9% stake in the company and, as with GameStop, made public business recommendations and picked several members of Bed Bath & Beyond’s board. Cohen’s main proposal for Bed Bath & Beyond was that the company should sell its one bright spot, its subsidiary buybuy BABY, which sells items for babies and children. As with GameStop, Bed Bath & Beyond’s stock price rose and became a popular meme stock, despite the company’s well-known struggles.
But by August 2022, Bed Bath & Beyond’s leadership had decided against selling buybuy BABY. Instead, the company planned to use the subsidiary as collateral to borrow more money, an agreement finalized in late August 2022. At the same time, Bed Bath had announced more bad news, firing 20% of its workforce and closing 150 stores.
But before all that became public, Plaintiffs allege, Cohen hatched a plan to profit from his huge investment in Bed Bath & Beyond. As alleged in their complaint, starting in early August 2022, Cohen made three moves designed to drive Bed Bath & Beyond’s stock price higher so that Cohen could sell his stake at a profit.
First, Cohen tweeted an emoji. On August 12, 2022, CNBC.com tweeted a negative story about Bed Bath & Beyond accompanied by a picture of a woman pushing a shopping cart in one of the Company’s stores. Cohen fired back with a tweet saying, “At least her cart is full,” which he capped with an emoji of a “smiley moon.”
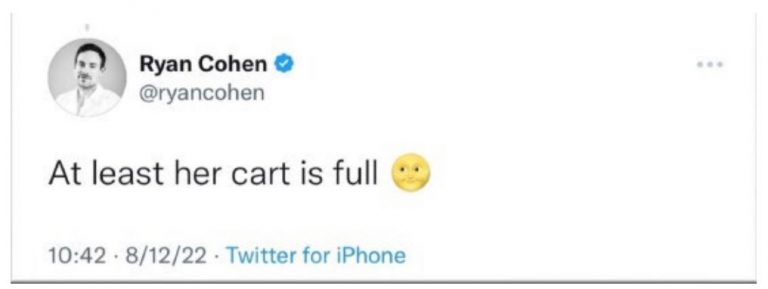
Many meme stock investors interpreted Cohen’s smiley moon emoji to mean “to the moon” or “take it to the moon,” a phrase that meme stock investors commonly use when they are predicting a stock price to increase. The complaint alleges that Cohen used the tweet to tell his thousands of meme stock investor followers that Bed Bath’s stock was about to rise and that they should either buy or hold their positions. And they appeared to act on his tip. Bed Bath’s stock price soared.
Four days later, Cohen filed a Schedule 13D document with the SEC stating that he had not recently sold any Bed Bath Stock. If Cohen had any concrete plans to sell his stock, he was legally required to disclose those plans on his Schedule 13D, but Cohen mentioned no such plans. Meme stock investors saw this as even more evidence that Cohen remained enthusiastic about Bed Bath’s growth prospects and its stock price continued to rise.
Finally, later that same day, Cohen filed a Form 144 with the SEC, which outlined his potential plan to sell his stock. But at that time, Cohen could file his Form 144 on paper via email, so his Form 144 was not immediately made public.
Meanwhile, over two days, on August 16 and 17, Cohen quietly sold his entire stake in Bed Bath & Beyond for a whopping profit of $68 million. When news finally broke that Cohen had sold off his entire stake, Bed Bath’s stock plunged by more than 50% within a few days.
Moving to dismiss the complaint, Cohen claimed that emojis can never be actionable because they have no defined meaning, asserting that there is no way to establish the truth of “a tiny lunar cartoon.”2 Judge McFadden rejected that argument, explaining that emojis are “symbols” that are an “effective way of communicating ideas” and “[e]mojis may be actionable if they communicate an idea that would otherwise be actionable.”3 Judge McFadden put it simply: “A fraudster may not escape liability simply because he used an emoji.”4
In this case, Judge McFadden explained, the complaint plausibly alleged that the smiley moon tweet relayed Cohen’s communication to his followers that Bed Bath & Beyond’s stock price was going up and that they should buy or hold.
Judge McFadden rejected most of Cohen’s other arguments as well. Cohen argued that the Complaint did not adequately allege “scheme liability” under Section 10(b) of the Exchange Act, claiming that scheme liability claims cannot be based “solely upon misrepresentations or omissions.” But Judge McFadden explained the Complaint alleged “a pump and dump scheme that relies on more than just misrepresentations or omissions,”5 including Cohen’s delayed filings of two SEC forms. Judge McFadden also refused to dismiss the Plaintiff’s insider trading claims under Section 20A and its market manipulation claims under Sections 9(a)(3) and 9(a)(4), providing important precedent for claims that are rarely litigated.
Six weeks after Judge McFadden’s decision, The Wall Street Journal reported that the SEC was investigating Cohen about his ownership and trades of Bed Bath & Beyond stock, making clear the significance of Cohen’s alleged misconduct.
- Cohen Milstein filed the first amended complaint in the case and currently serves as Liaison Counsel to the proposed class.
- In re Bed Bath & Beyond Securities Litigation, 1:22-cv-02541, ECF No. 91, at 10 (D.D.C. July 27, 2023).
- Id.
- Id. at 10-11.
- Id. at 22.
- Id.
By Kate Fitzgerald
Plaintiffs in an antitrust lawsuit accusing a handful of prime broker banks of colluding to keep prices in the stock loan market artificially high have received initial approval for a settlement requiring the banks to pay nearly $500 million in cash and make reforms that should reduce the chances of collusion in the future.
On September 1, 2023, the Hon. Katherine Polk Failla of the United States District Court for the Southern District of New York granted preliminary approval of plaintiffs’ class action settlement with four Defendant banks—Morgan Stanley, Goldman Sachs, UBS, and JP Morgan—and with EquiLend, the securities lending trading platform Defendants control. According to Plaintiffs, the Defendant banks conspired through EquiLend since at least 2009 to keep markets opaque and thwart modernization, thereby keeping prices artificially high.
Counting the $499 million cash component of the latest settlement, Plaintiffs have now recovered $580 million from Defendants, pending final approval. An $81 million settlement with Credit Suisse received preliminary approval last year.
Filed in 2017, Iowa Public Employees’ Retirement System, et al. v. Bank of America Corp. et al. is led by five institutional investors, including four public pension funds, represented by Cohen Milstein and its co-counsel. The Plaintiffs—Iowa Public Employees’ Retirement System, Los Angeles County Employees Retirement Association, Orange County Employees Retirement System, Sonoma County Employees’ Retirement Association, and Torus Capital LLC—asserted that the banks’ actions to preserve their market dominance violated federal antitrust laws, causing market participants financial harm. The Plaintiffs sought financial damages and improvements to the system.
The $1.7 trillion stock loan market is a critical component of global securities markets, facilitating activities like short selling and hedging while providing a stream of income to beneficial owners who lend out their securities. By temporarily lending stocks to another entity, typically for a fee, long-term investors who hold large amounts of publicly traded securities can generate additional income for their portfolios. The borrowing entities, in turn, are able to borrow stocks they need to enable short sales and hedging strategies.
But, as alleged in the complaint, the institutional investors who lend and borrow stocks believe that, for years, they were forced to use an inefficient, antiquated, and opaque over-the-counter trading platform which forced market participants to use defendant Prime Brokers as middlemen to match buyers and sellers for a fee, which Defendants allegedly conspired to keep the market frozen in its inefficient state to preserve their collective market control and dominance and charge higher transactional fees.
In their complaint, Plaintiffs allege that since at least 2009, the six Defendant banks routinely took steps together to block the development of competitive exchange platforms in the stock loan market, like AQS (in the United States) and SL-x (in Europe)—exchanges that would have reduced trading costs for both stock lenders and borrowers. For example, the Complaint alleged that when the banks learned that Bank of New York (BONY) was using AQS for stock loan transactions, Goldman Sachs threatened to return billions in collateral and never do business with BONY again. BONY promptly abandoned its plans. Various Defendants took similar steps with well-known hedge funds, too—SAC Capital, Renaissance Capital, and others—telling them they would not connect them to AQS, and, if they did not like it, they could take their business elsewhere.
In 2001, the six prime broker banks, together with four others, created EquiLend, a securities lending platform and dealer consortium purportedly created to enhance market efficiencies in the stock loan market. The board of directors of EquiLend consisted of a representative from each Defendant bank, something that plaintiffs allege helped them control and protect their profits in the stock loan market.
The Complaint alleged that through EquiLend, the banks could collectively agreed not support any exchange that would permit borrowers and lenders to trade directly with each other in a modern all-to-all market.
In the Complaint, Plaintiffs contend that in 2016 alone these six banks skimmed approximately 60% of the $9.15 billion in stock lending revenue, despite performing a service for which they bear virtually no risk. Any other arrangement would have substantially reduced the need for their services, and the premiums that they charged would have been untenable.
The Complaint alleges that after boycotting securities lending participants who participated on other platforms—AQS in the US and SL-x in Europe— the banks either purchased the intellectual property underlying those exchanges (SL-x) or the exchange itself (AQS), effectively shelving the efforts to improve stock lending for investors. The purchase of AQS by bank-controlled EquiLend—the last piece of the conspiratorial puzzle because it gave the banks complete control over all gateways to central clearing in the US—even had a secret code name at Morgan Stanley: Project Gateway.
After years of painstaking and costly discovery, in February 2022, Credit Suisse became the first of the six banks to settle. Morgan Stanley, Goldman Sachs, UBS, JP Morgan, and EquiLend followed suit in September 2023.
While the settling Defendants have denied any wrongdoing and say reforms are unnecessary, Plaintiffs believe that the equitable relief they designed and negotiated for will help align EquiLend to the best practices and guidelines for anti-cartel and collaborations among competitors.
These reforms include:
- Mandatory rotation of outside antitrust counsel and EquiLend board members;
- Limitations on who can access commercially sensitive information; and
- A robust compliance, training, and monitoring program at EquiLend.
At least one industry observer is cautiously optimistic about the settlement’s injunctive relief. In a recent article, financial investor publication Pensions & Investments said that the terms of the settlement “may bring the first bit of transparency to stock lending.” The article noted, however, that many of the case documents that could shed further light on the inner workings of stock loan market remain under judicial seal.
Cohen Milstein and co-counsel continue to pursue the case against Bank of America, the only remaining Defendant bank.
Even as avenues for consumers to pursue group litigation abroad expand, activity in shareholder lawsuits outside the United States has grown more narrowly focused on jurisdictions with lower adverse risks and better histories of recovery, according to recent publications by service providers.
While the number of non-US shareholder actions filed in the first half of this year held steady, “litigation funders have changed their geographic focus, investing more resources in countries perceived as lower risk with more expedited legal proceedings and away from countries where previously filed suits have been slower and more challenging than expected,” Financial Recovery Technology (FRT), a global class action recovery service, wrote in its Mid-Year 2023 in Review.
According to FRT, 25 recovery actions were initiated outside the US from January through June, nearly on track to match the 52 cases filed in 2022. The 2023 cases were filed across six jurisdictions, however, compared with 11 countries in 2022. Both totals include opt-in actions, where affected investors are required to register as parties relatively early in the proceedings, as well as opt-out actions, where (like in US federal class actions) affected shareholders can wait until a case is decided to file a settlement claim.
The review found that countries with opt-out actions, such as Australia and the Netherlands, were seeing an uptick in new matters, while Germany, Brazil, and other jurisdictions where prior cases have become bogged down have seen fewer new filings. Risk calculations were also driving institutional investors’ decisions on whether to register for cases, FRT said, adding that, so far in 2023 it has seen “the greatest client participation in passive [opt-out] countries including Australia and the Netherlands.”
ISS Securities Class Action Services (SCAS), another settlement-claims-filing service, also underscored the importance of assessing adverse cost risks in overseas litigation, even when third-party litigation funders take out “after the event” (ATE) insurance—policies designed to kick in if a court orders plaintiffs to pay defendants’ costs.
“[E]ven with litigation funding and ATE insurance, there is still a risk that participating investors could be left footing some of the bill,” SCAS wrote in a September 2023 white paper, Participating in Securities Collective Actions Outside the US: Are Adverse Costs Worth the Risk? In one English High Court case where the judge found for defendants in 2019 (Lloyds/ HBOS), defendants claimed more than £30 million in legal costs, £9 million more than that funder’s ATE insurance cap.
The white paper highlighted the risk of paying adverse costs in five countries, saying risks were lowest in Australia, the Netherlands, and Japan and highest in the United Kingdom and Brazil. Despite such generalizations, the paper’s authors said investors need to rely on a “thorough, objective case and jurisdictional analysis” to make decisions on whether to participate in non-US litigation.
“Unfortunately, there is no universal approach to handling adverse costs across multiple jurisdictions,” they wrote. “The adverse cost risk is highly contextspecific based on the jurisdiction, the particular case, and the terms offered by the funder.”
If new cases and participant registrations are concentrated in a handful of lower-risk countries, the EU and the UK, at least, continue to expand their own legal mechanisms for collective redress. Depending on the commentator, this process is either leading to an explosion of “US-style” class actions or a more measured, European model.
“The growth of group litigation in the UK and Europe over recent years has been exponential, and its significance to businesses as a key corporate risk will only continue to increase,” the law firm Jones Day wrote in an October 2023 client booklet, The Rise of US-Style Class Actions in the UK and Europe.
In the UK, for example, Jones Day said 13 new claims were brought in 2022 under the collective proceedings order introduced in 2015, double the number in 2021. The authors attributed the rise to a “confluence” of factors: “an upswing in the thirdparty litigation funding market, increasingly sophisticated and experienced claimant law firms, and liberalised group claim procedures.”
Still, the UK has been a bit of a bumpy road for case organizers and funders. Most recently, the UK’s Supreme Court ruled that litigation funding agreements that provide funders a share of damages should be defined as “damages-based agreements,” meaning that they are unenforceable unless comply with a strict set of regulations that, among other things, limit the percentage to 10 percent— and completely unenforceable for opt-out proceedings before the Competition Appeal Tribunal. In the wake of this agreement, funders are working to revamp their agreements with registrants.
Unlike the UK, the EU appears to be moving slowly and steadily toward more claimant-friendly “collective redress” procedures. The European Commission’s 2018 “New Deal for Consumers” and 2020 “Representative Actions Directive” required the EU’s 27 member states to put in place a collective action mechanism for consumers in a range of sectors, including data protection, financial services, travel and tourism, energy, and communications. These cases can be filed by “qualified entities”— typically consumer groups registered in at least one of the EU countries—that represent a minimum number of claimants.
While adoption has been slow enough to prompt the European Commission to send “formal notice” in January to member countries who had failed to change their laws to conform, at least 10 countries have now complied with the directive, with all but a few in the process of doing so.
On September 29, 2023, Germany became the latest EU member country to approve a law transposing the Representative Actions Directive. Expected to take effect by the end of October, the new law expands protections for consumers in German courts, where previous collective actions provided only declaratory and injunctive relief. Also under the new law, consumers will have until three weeks after the end of oral proceedings to register their claim, when they will have a better idea of the case’s chances for success.
That said, the German parliament estimates that only 15 cases a year will be brought under the new law. Bird & Bird attorney Susanne Lutz says that is because of features in the final law that favor defendants. For one thing, at least 50 impacted consumers need to group together to commence an action. For another, litigation funding fees are capped at 10% of the “economic benefit resulting from the redress action” and contractual arrangements must be disclosed. In addition, the law dropped a proposal to include disclosure obligations for relevant documents. And finally, claimants can still be found liable for adverse costs, though the €300,000 cap is less than originally proposed.
“Many companies feared the introduction of a ‘class action’ based on the US model; however, this fear is not justified,” she wrote, adding that representative actions brought under the law “should not be able to be utilized as a business model for purely profit-making purposes.”
The future of pharmaceutical antitrust litigation will likely focus in large part on biologics and biosimilars. Cases involving the delayed market entry of generic pharmaceuticals have traditionally focused on small-molecule generic drugs.
In recent years, though, the U.S. drug market has come to be dominated by biologics. Biologics — and their generic counterparts, called biosimilars — are prescription drugs derived from living cells; examples include insulin, vaccines and antibody drugs.[1]
Following five years of double-digit annual growth, in 2022 biologics captured 46% of U.S. prescription drug spending — approximately $261 billion.[2]
Generic-delay cases involving small-molecule drugs are familiar territory for many pharmaceutical antitrust litigators, though the same is not yet true for biosimilar-delay cases.
Whereas small-molecule generics have been regulated by the Hatch-Waxman Act for nearly 40 years, biosimilars have been authorized under U.S. law for just over a decade. And new evidence indicates that while biosimilar markets are largely akin to small-molecule generic markets, there are also important differences.
Those differences have been well illustrated by 2023’s launch of biosimilar versions of Humira. Humira was the single-largest line item in the 2022 U.S. pharmaceutical budget.[3] Americans spent over $18 billion on the biologic, which treats conditions such as rheumatoid arthritis.[4]
Why the high prices? Humira’s manufacturer, AbbVie Inc., long maintained a monopoly on the drug. In mid-2023, however, a wave of biosimilar Humira competitors finally came to market, with the most recent launching in October.[5]
This article identifies the emerging ways in which biosimilar markets differ from traditional small-molecule drug markets, and recommends how pharmaceutical antitrust litigators can account for these market dynamics in biosimilar-delay cases.
Generic-Delay Cases and Biosimilars: Overview
Generic-delay litigation involves claims that pharmaceutical companies have improperly delayed the market entry of lower-priced generic versions of a drug, thereby causing payers — e.g., wholesalers, self-insured employers or patients — to overpay for a period of time.
Perhaps the most well-known type of generic-delay case is the “reverse-payment,” or “pay-for-delay,” case, which was recognized by the U.S. Supreme Court in FTC v. Actavis.[6]
Pharmaceutical companies can also face liability for other misconduct that delays generic competition, such as abusing the Orange Book system,[7] refusing to sell essential inputs to prospective generic competitors,[8] or filing sham patent litigation.[9]
Generic-delay cases have traditionally focused on small-molecule, chemically derived drugs — which are governed by the 1984 Hatch-Waxman Act. But an increasingly large share of prescription drug payments now goes toward biologics. Biologics are derived from living cells, and their therapeutic equivalents are called biosimilars.
The statute authorizing biosimilars — the Biologics Price Competition and Innovation Act — was enacted in 2010.[10]
The biosimilar market is thus relatively nascent: Whereas the U.S. Food and Drug Administration approved 722 small-molecule generic drugs in 2022,[11] it has approved a total of just 43 biosimilars since the BPCIA was enacted in 2010.[12]
Despite their relative nascency, biologics and biosimilars now account for approximately 46% of U.S. prescription drug spending — $261 billion per year.[13]
With such substantial revenues at stake, pharmaceutical companies have strong motivations to use anti-competitive tactics to delay the onset of biosimilar competition, just as they have to delay the entry of small-molecule competition.
Differences between small-molecule generic markets and biosimilar markets, however, may warrant special attention from practitioners, as the same litigation strategies that have successfully policed small-molecule delay cases may require adjustment in biosimilar cases.
Same-Tier Formulary Coverage
Payers, and pharmacy benefit managers acting on their behalf, use several tools to incentivize the use of lower-priced generic drugs rather than more expensive branded drugs. One tool is the formulary, a list that organizes drugs into tiers, which render drugs more or less expensive for plan members.
For example, a formulary may impose a $10/$30/$50 copay for drugs on the first/second/third tiers, with generic drugs usually on the least-expensive first tier.[14] The formulary and other mechanisms[15] have contributed to small-molecule generics rapidly capturing the vast majority of brands’ market share in competitive generic markets.[16]
Despite biosimilar versions of Humira carrying a markedly lower list price than the branded product, many biosimilars are nonetheless being placed on formulary tiers equal to those of the name brand.[17] Placing biosimilars on the same formulary tier as their branded counterparts means that biosimilars may not capture the same high level of market shares as do small-molecule generics, as patients will not be incentivized to use the biosimilar by the promise of lower copays.
If biologics do maintain a greater share following biosimilar entry — as appears to be occurring with Humira[18] — one consequence in biosimilar-delay cases will be an increased importance of brand-brand damages.
As with small-molecule drugs, biosimilar competition can drive down the price of the branded drug compared to what the name brand’s price would be without competition.[19] Thus, even where payers would have purchased the branded drug had competition not been improperly delayed, they would have paid less for it and were thus damaged. These are called brand-brand damages.[20]
Practitioners should expect that brand-brand damages may be a meaningful part of the damages claims in biosimilar-delay cases, and be sure to prepare the fact and expert discovery necessary to establish, or respond to, claims for brand-brand damages.
One Biosimilar, Two Prices
Another unique dynamic that has appeared in biosimilar markets, including for Humira, is companies launching the same product at two different price points: one with a high list price and substantial rebates, and a second with a lower list price but few or no rebates.
Biosimilar versions of Humira have generally coalesced around 5% off and 85% off Humira’s list price — just under $7,000 for a month’s supply as of 2023 — with multiple companies offering both a high-list price and low list-price version.[21]
For example, Boehringer Ingelheim Vetmedica Inc.’s Oct. 3 launch of an unbranded biosimilar at an 81% discount follows its July 2023 launch of a branded biosimilar priced at a 5% discount.[22]
A post-generic entry pricing regime with multiple list prices will affect how plaintiffs calculate their damages in biosimilar-delay cases. In small-molecule delay cases, experts generally identify what the name-brand price is and what the generic price would have been at a given time in the but-for world absent the defendants’ anti-competitive conduct.
Identifying a generic price is possible because small-molecule generics in competitive markets tend to coalesce closely around a prevailing price.[23] Unlike in small-molecule cases, however, the launch of biosimilar Humira products shows that there will be biosimilars marketed with markedly different list prices.
Plaintiffs might address this dynamic in different ways. One could be to group together the name brand with the high-priced biosimilars as being the branded price and consider the lower-priced drugs as representing the biosimilar price.
It is no concern that one company may offer two differently priced products; branded manufacturers already do this with small-molecule drugs in marketing authorized generics.
From this perspective, biosimilar markets resemble small-molecule markets with the only difference being that multiple products are marketed around the name brand’s price point instead of only one.
Another approach could be to model the market as having three price points: a branded price, a high-priced biosimilar and a low-priced biosimilar.
While requiring an additional layer of analysis as compared to the first approach, this carries the benefit of acknowledging that even the high-priced biosimilars are marketed at a slight list-price discount to the brand — in Humira’s case approximately 5%.
Whatever strategy is ultimately taken, practitioners should be mindful that biosimilar market pricing structures differ from small-molecule markets. Counsel should coordinate early in the case with experts to understand the different biosimilar players and their prices, and develop a strategy for modeling the prices that payers would have paid in the but-for world absent the anti-competitive conduct.
Product-Specific Differences
While small-molecule generics can be automatically substituted at pharmacies for their branded counterparts, the same is true of biosimilars only when they have received an interchangeable designation.[24] As of October 2023, only two Humira biosimilars have received such a designation.[25]
There are thus product-specific differences among the biosimilars which one might not typically see in small-molecule generics. For example, some, but not all, Humira biosimilars contain citrate — an ingredient that can cause pain at the injection site. Some, but not all, Humira biosimilars are marketed in a high-concentration formula, which accounts for about 85% of prescriptions.[26]
Importantly, none of these differences should take the products outside of the relevant antitrust market — i.e., they all compete on price and will drive down overall spending on these drugs. Indeed, in a recent earnings call, AbbVie CEO Richard Gonzalez said the company is “competing very effectively with the various biosimilar offerings.”[27]
Nonetheless, defendants may attempt to seize on such product-specific differences to resist efforts to hold them accountable for anti-competitive conduct.
For example, defendants in private class action litigation could seek to argue that the product differences limit plaintiffs’ abilities to demonstrate elements of their case on a classwide basis.
Defendants could also attempt to argue that variation among biosimilars means the products do not fall within the same relevant antitrust market.
Practitioners should be prepared to account for these product-specific differences in addressing issues of market power, classwide proof and otherwise throughout the litigation. In order to address these issues, practitioners should consider retaining a medical expert.
Medical experts, who are often, though not always, used in generic-delay cases, may be especially crucial in providing the fact finder with expert analysis on why these product-specific differences do or do not matter for a party’s positions.
Conclusion
The emergence of biosimilars is a beneficial development for the U.S. health care system, as these affordable medicines stand to save Americans approximately $180 billion over the next five years.[28]
Thus, cases policing anti-competitive delay of biosimilar competition will remain an important tool for antitrust enforcers to promote competition in health care markets.
However, practitioners should remain mindful that biosimilar markets are relatively nascent, and successful enforcement of the antitrust laws against companies that improperly delay biosimilar competition will require thoughtful consideration of the specific dynamics that prevail in the emerging biosimilar market.
Read Lessons For Biosimilar and Biologic Antitrust Litigation.